To put my thoughts in order, I’ve decided to summarise my view on type 2 reversal and what it means.
Type 2 Diabetes Mellitus (T2DM) is often considered a life-long, irreversible, progressive disease. People newly diagnosed are told that that’s it, they have diabetes for the rest of their life, and it will only get worse. For most people it’s crushing news and many go into denial of the condition: if they can’t stop it or reverse it, there is no reason to do anything about it. Just wait for death.
This is a depressing situation not prone to people making the efforts they need to make to improve their situation.
A note on vocabulary before we continue:
– I’m not talking about “cure” for T2DM. The word cure is too emotionally charged and isn’t conductive of good debate.
– The aim of treatment is to reach a state of “post-diabetes”, aka remission. It’s important to understand that the purpose of the process isn’t to go back to a pristine body that has never been exposed to diabetes. It’s most likely impossible since damage has been done during diabetes onset. For example, it’s not clear whether muscle insulin resistance can be completely reversed. But it doesn’t seem to be determinant in the natural history of T2DM. Post-diabetes refers to a state of the body where it behaves functionally like a non-diabetic body, no matter what it’s been through historically.
– Remission is a word that people know from diseases such as cancer. It implies that the disease can come back out of the blue and therefore it is never cured for sure. Research suggests that it is not the case for diabetes. I therefore prefer to use the term “post-diabetes”.
– I’ll call the process needed to reach post-diabetes “reversal”. Reversal doesn’t mean that the body is put back into a pre-diabetes development state. It reflects the change of direction in the metabolic process that leads to T2DM. It implies that in the end the body’s function is reversed to a functionally non-diabetic state, i.e., for all intents and purposes, it behaves like a normal body (HbA1c<6.5% for at least 3 months and 2 separate tests, fasting glucose levels<100mg/dl, normal glucose tolerance, no medication) with reduced complications and risk factors.
The message that doctors should give their newly diagnosed patients is that their body has a problem that results in high glucose concentration in the blood. That high glucose concentration has side-effects that have many consequences, from headaches, to neuropathy, to increased heart disease risk, and can result in higher holiday insurance, eye sight loss, limb amputation, and even death.
Respected organizations like the World Health Organization and Diabetes UK now openly acknowledge that diabetes is metabolically reversible. It is not even clear anymore whether the condition is really progressive as many of the studies that led to that claim were studying populations who were gaining weight, year on year (e.g. [7][8]). In fact, the health services in the UK (NHS) and in Australia, having reproduced the results of some of the studies, have decided to implement their protocols on a large scale for their diabetic patients. But that hasn’t reached most general practitioners who still follow their (light) training on diabetes and tell their patients they’re doomed.
Medication is more and more available to stabilise the effects of the condition, but it isn’t sufficient long term in a lot of cases because they don’t remove the root cause of the disease. Using medication without lifestyle changes often leads to a progressive disease [22] and only delays the long-term effects of diabetes. It is what many patients want so that they can continue living their life as they have always done, but it isn’t the solution [21].
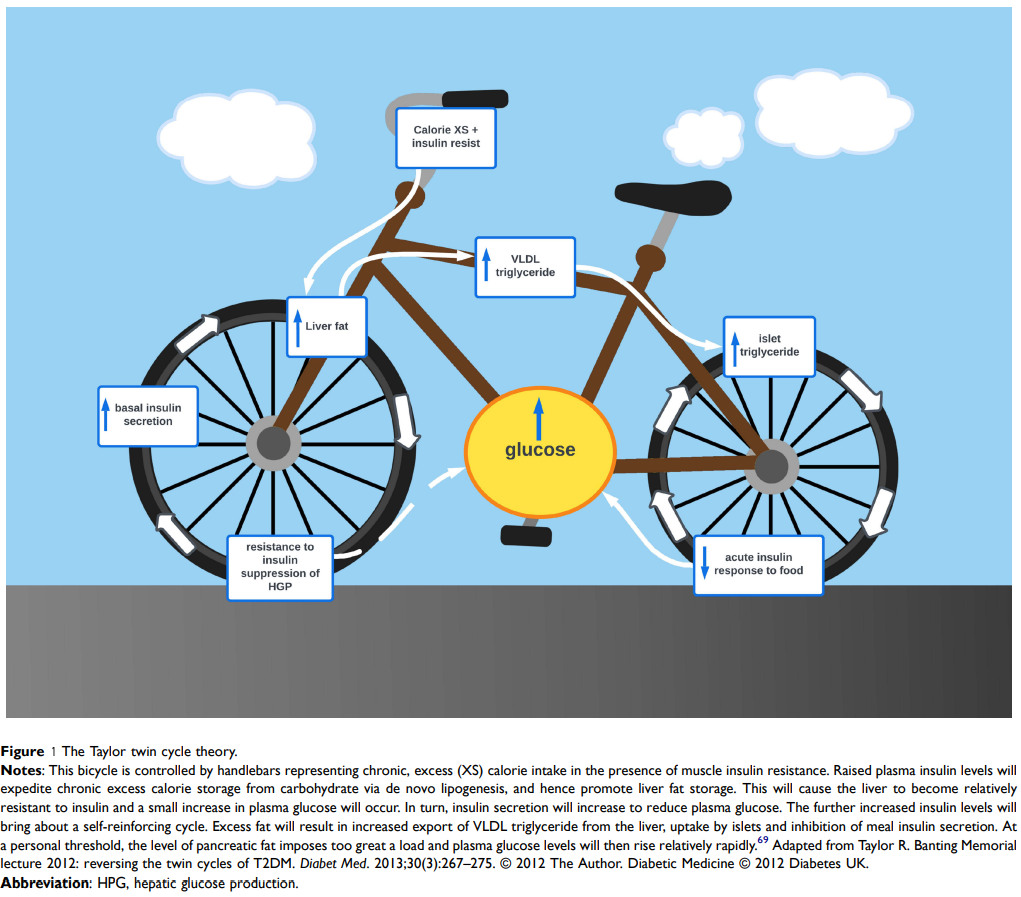
The mechanism that leads to the development of diabetes isn’t well understood yet. Dr Roy Taylor has developed the “Twin Cycle Hypothesis” [18] (fig 1) to explain the mechanism that leads to diabetes [1]. It makes sense to me so I adopted it as working hypothesis. When glucose is absorbed (as sugar or carbohydrates), it is stored first as glycogen, the fuel for muscles. That glycogen can be converted back into glucose when the muscles need it. However, glycogen storage is very limited, so the excess glucose is stored as fat in adipose tissue. That’s the only way glucose can be dealt with in the body. However, that storage also has its limits, and eventually, continued exposure to excess glucose leads to storage of fat in liver and pancreas. Liver and pancreas are interlinked in the management of blood glucose. Pancreas secretes insulin from its Beta cells that instructs muscles and liver to store glucose (e.g., after a meal). The constant exposure to excess glucose will trigger calorie storage via de novo lipogenesis, and hence promote liver fat storage. As this process is controlled by insulin and T2 diabetics tends to have an inherent higher level of insulin resistance, that process is exacerbated as insulin secretions increase. In return, the storage of fat in the pancreas produces endoplasmic reticulum stress that can bring about dedifferentiation and loss of specialised function (insulin production). This creates a vicious cycle of insulin resistance, hyperinsulinemia, and fat deposition in the liver [20] which leads to diabetes, i.e. increased blood glucose.
Recent studies (e.g. bariatric surgery follow-ups [5][6][10], LookAHEAD [4], Counterpoint [2], DiRECT [3]) have therefore shown that the key to reversing diabetes is to remove accumulation of inappropriate fat from organs such as liver and pancreas. That removal allows expression of the insulin gene and redifferentiation of Beta cells. This can be done through significant weight loss. The DiRECT study puts the minimum threshold at about 15kg. The Counterpoint study at 10% of body weight with a drop in beneficial gain at about 20% of body mass.
Weight loss doesn’t even mean that you have to reach a healthy BMI. Researchers talk about “personal fat threshold”, i.e. the level of body fat above which the body no longer stores additional fat in an appropriate way. That personal threshold varies from person to person [15] [16], hence the reason why BMI isn’t a good indicator of what level will trigger diabetes. And indeed 72% of people with a BMI > 40kg/m2 don’t have diabetes. The purpose of the weight-loss process is therefore to bring the body below that threshold so that normal fat storage can resume and the liver and pancreas are freed from inappropriate fat storage.
There are many factors that will make it harder or impossible to achieve post-diabetes. For example:
– Age. With age, insulin resistance tends to increase. After a certain age, it becomes very difficult to reverse diabetes (maybe 65 years old).
– Ethnicity. Asian and to a lesser extent African ethnicity are more prone to diabetes and will find reversing it more difficult.
– Time since diagnosis. The more time has passed since the onset of diabetes, the harder it becomes to reverse it. The Counterpoint study placed the threshold at 10 years, the DiRECT study at 8. Beyond that time, patients have managed to bring their body to pre-diabetes levels, but never to non-diabetic.
– Personal fat threshold. Some people will develop diabetes without being overweight. For them, weight loss can still lead to reversal, but those who can’t lose much weight could find it impossible.
There is therefore no guarantee that following the guidelines regarding weight loss will bring successful reversal.
Once achieved, how long reversal remains in place is not clear yet. Bariatric surgery follow-ups have shown that it can last at least 10 years [9] or even 15 years [19] with long term stable remission rates of about 30%. More recently, the LookAHEAD study had 5 [12] and 8 [13] years follow-ups, the DiRECT study has had 12 months [11], 3 years, and 5 years [14] follow-ups, that showed that some of the patients maintained their post-diabetes status during those years. As long-term studies take time and are expensive, little data is currently available. But it seems that it can be maintained over many years providing that no weight gain takes place.
It is to be noted that there is no inevitability in Beta cell deterioration after adequate weight loss and during weight stability [17]. That is encouraging, because it means that T2DM isn’t progressive. There is no certainty that eventually diabetics will require insulin or medication if they achieve post-diabetes.
Many will call this “control”, especially in diabetes support groups e.g. on Reddit. That’s wrong and that’s only a way to minimise the effect of this process so they don’t have to do it themselves. Control implies that some long-term process is put in place to manage the symptoms of diabetes and keep the condition in check. The reversal process only requires long term weight stabilisation after the initial weight loss. There is no need for any particular control, no specific diet, no process. Simply food intake that doesn’t lead to weight gain.
Indeed, as muscle insulin resistance might not change through the weight-loss process, it is extremely likely that weight-gain, and especially a return to original weight, will bring back the condition. Avoiding weight-gain is therefore the absolute priority of the post-diabetic phase.
It is still not accepted widely among diabetics, but I think that state of research is very encouraging and matches my personal experience so far.
Citations:
[1] “Can type 2 diabetes be reversed and how can this best be achieved? James Lind Alliance research priority number one”, R Taylor , A C Barnes, Diabet Med. 2019 Mar;36(3):308-315. doi: 10.1111/dme.13851. Epub 2018 Dec 3.
[2] “Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol”, E. L. Lim,1 K. G. Hollingsworth, B. S. Aribisala, M. J. Chen, J. C. Mathers, and R. Taylor, Diabetologia. 2011; 54(10): 2506–2514. doi: 10.1007/s00125-011-2204-7
[3] “Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial”, Michael Ej Lean, Wilma S Leslie, Alison C Barnes, Naomi Brosnahan, George Thom, Louise McCombie, Carl Peters, Sviatlana Zhyzhneuskaya, Ahmad Al-Mrabeh, Kieren G Hollingsworth, Angela M Rodrigues, Lucia Rehackova, Ashley J Adamson, Falko F Sniehotta, John C Mathers, Hazel M Ross, Yvonne McIlvenna, Renae Stefanetti, Michael Trenell, Paul Welsh, Sharon Kean, Ian Ford, Alex McConnachie, Naveed Sattar, Roy Taylor, Lancet. 2018 Feb 10;391(10120):541-551. doi: 10.1016/S0140-6736(17)33102-1. Epub 2017 Dec 5.
[4] “Association of an intensive lifestyle intervention with remission of type 2 diabetes”, Edward W Gregg, Haiying Chen, Lynne E Wagenknecht, Jeanne M Clark, Linda M Delahanty, John Bantle, Henry J Pownall, Karen C Johnson, Monika M Safford, Abbas E Kitabchi, F Xavier Pi-Sunyer, Rena R Wing, Alain G Bertoni; Look AHEAD Research Group, JAMA. 2012 Dec 19;308(23):2489-96. doi: 10.1001/jama.2012.67929.
[5] “Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis”, Buchwald H, Estok R, Fahrbach K, et al., Am J Med. 2009;122(3):248–256. doi:10.1016/j.amjmed.2008.09.041
[6] “Bariatric surgery. A systematic review and meta-analysis”, Buchwald H, Avidor Y, Braunwald E, et al., JAMA. 2004;292(14):1724–1737. doi:10.1001/jama.292.14.1724
[7] “Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease”, U.K. Prospective Diabetes Study Group. Diabetes 1995;44:1249–1258.
[8] “Natural history of pancreatic islet B-cell function in type 2 diabetes mellitus studied over six years by homeostasis model assessment”, Rudenski AS, Hadden DR, Atkinson AB, Kennedy L, Matthew DR, Merrett JD et al., Diabet Med 1988; 5:36–41.
[9] “Surgical treatment of obesity and its effect on diabetes: 10-y follow-up”, Pories WJ, MacDonald KG, Jr, Morgan EJ, Sinha MK, Dohm GL, Swanson MS et al., Am J Clin Nutr 1992;55:582S–85S
[10] “Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial”, Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S et al., JAMA 2008;299:316–323
[11] “Calorie restriction and reversal of type 2 diabetes”, Taylor R., Expert Rev Endocrinol Metab 2016;11:521–528
[12] “Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes”, Paisey RB, Frost J, Harvey P, Paisey A, Bower L, Paisey RM et al., J Hum Nutr Diet 2002;15:121–127
[13] “Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study”, LookAhead, Obesity 2014;22:5–13
[14] “Weight loss can put type 2 diabetes into remission for at least 5 years, direct study reveals”, Diabetes UK, 2023, diabetes.org.uk/about_us/news/weight-loss-can-put-type-2-diabetes-remission-least-five-years-reveal-latest-findings
[15] “The genetics of lipid storage and human lipodystrophies”, Robbins AL, Savage DB, Trends Mol Med 2015;21:433–438
[16] “Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome – an allostatic perspective”, Virtue S, Vidal-Puig A., Biochim Biophys Acta 2010;1801:338–349
[17] “Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery”, Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS et al., Cell Metab 2018;28:1–10
[18] “Pathogenesis of Type 2 diabetes: Tracing the reverse route from cure to cause”, Taylor R., Diabetologia 2008;51:1781–1789
[19] “Association of bariatric surgery with long-term remission of T2DM and with microvascular and macrovascular complications”, Sjostrom L, Peltonen M, Jacobson P, et al., JAMA. 2014;311:2297–2304. doi:10.1001/jama.2014.5988
[20] “Serial metabolic measurements and conversion to T2DM in the west of Scotland coronary prevention study: specific elevations in alanine aminotransferase and triglycerides suggest hepatic fat accumulation as a potential contributing factor”, Sattar N, McConnachie A, Ford I, et al., Diabetes. 2007;56:984–991. doi:10.2337/db06-1256
[21] “Pills are not the answer to unhealthy lifestyles”, Godlee F., BMJ. 2018;362:k3046. doi:10.1136/bmj.k3046
[22] “UK prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease”, Diabetes. 1995;44(11):1249–1258. doi:10.2337/diab.44.11.1249